Genetics in Hypertrophic Cardiomyopathy (HCM)
Genetics in Hypertrophic Cardiomyopathy (HCM)
1. What is the most common mode of inheritance in Hypertrophic Cardiomyopathy?
A. Autosomal dominant
B. Autosomal recessive
C. X-linked recessive
D. Mitochondrial inheritance
💬 Explanation: HCM is typically inherited in an autosomal dominant fashion, meaning one copy of the mutated gene is sufficient to cause disease.
2. Which gene is most commonly mutated in HCM?
A. MYBPC3
B. ACTN2
C. LMNA
D. DMD
💬 Explanation: MYBPC3 is the most commonly mutated gene in HCM, especially in Western populations.
3. Mutations in which of the following genes are associated with early-onset and more severe HCM?
A. MYBPC3
B. MYH7
C. PLN
D. SCN5A
💬 Explanation: MYH7 mutations often lead to more severe disease and earlier presentation.
4. Which sarcomeric protein is encoded by the MYH7 gene?
A. Cardiac troponin T
B. Myosin-binding protein C
C. β-Myosin heavy chain
D. α-Tropomyosin
💬 Explanation: MYH7 encodes the β-myosin heavy chain, a key sarcomeric protein in cardiac muscle contraction.
5. What type of mutation is commonly found in the MYBPC3 gene?
A. Frameshift only
B. Missense only
C. Truncating mutations
D. Splice site mutation exclusively
💬 Explanation: Truncating mutations are common in MYBPC3, often leading to haploinsufficiency and late-onset HCM.
6. What is the penetrance pattern of hypertrophic cardiomyopathy?
A. Complete and age-independent
B. Incomplete and age-dependent
C. Only in males
D. Recessive carriers only
💬 Explanation: HCM demonstrates incomplete and age-dependent penetrance, meaning not all mutation carriers will show symptoms, and expression can vary with age.
7. Which gene mutation is most associated with a higher risk of sudden cardiac death in HCM?
A. MYBPC3
B. MYH7
C. TNNI3
D. ACTN2
💬 Explanation: MYH7 mutations are more often associated with early onset, more severe hypertrophy, and increased risk of sudden cardiac death.
8. What type of mutation in MYBPC3 often leads to haploinsufficiency?
A. Missense
B. Truncating
C. Nonsense
D. Splice-site
💬 Explanation: Truncating mutations in MYBPC3 result in reduced functional protein, leading to haploinsufficiency and contributing to the phenotype of HCM.
9. What is the role of genetic testing in asymptomatic family members of HCM patients?
A. Not recommended
B. To identify carriers and initiate surveillance
C. For legal documentation only
D. Only if they are symptomatic
💬 Explanation: Genetic testing in asymptomatic family members helps identify carriers early so that regular follow-up and preventive care can be arranged.
10. Which of the following is a sarcomeric gene associated with HCM?
A. DMD
B. TNNT2
C. LMNA
D. PKP2
💬 Explanation: TNNT2 encodes cardiac troponin T, a sarcomeric protein. Mutations in this gene are known to cause HCM.
11. Which of the following best describes the function of sarcomeric proteins mutated in HCM?
A. Ion channel regulation
B. Hormonal signaling
C. Force generation and contraction
D. Lipid metabolism
💬 Explanation: The mutated proteins in HCM are sarcomeric proteins responsible for force generation during myocardial contraction.
12. A variant of uncertain significance (VUS) in HCM genetic testing means:
A. No mutation is present
B. Diagnosis is confirmed
C. The clinical significance of the mutation is not yet known
D. The variant is benign
💬 Explanation: A VUS is a gene variant whose effect on disease risk is not clearly established and may need reclassification as evidence evolves.
13. What is the utility of cascade screening in families with known HCM mutations?
A. Confirms the diagnosis in the proband
B. Identifies other at-risk family members
C. Rules out other cardiomyopathies
D. Guides pharmacotherapy
💬 Explanation: Cascade genetic screening helps identify asymptomatic relatives carrying the familial mutation for surveillance and early intervention.
14. Which mutation is typically associated with late-onset hypertrophic cardiomyopathy?
A. MYBPC3
B. MYH7
C. TNNT2
D. TNNI3
💬 Explanation: MYBPC3 mutations are often associated with milder forms and later onset of hypertrophic cardiomyopathy.
15. Which of the following genes is NOT typically involved in hypertrophic cardiomyopathy?
A. MYH7
B. MYBPC3
C. CFTR
D. TNNT2
💬 Explanation: CFTR is not related to HCM; it is associated with cystic fibrosis. HCM involves sarcomeric protein genes.
16. Which clinical tool is commonly used to monitor mutation carriers in HCM?
A. Holter monitor only
B. Echocardiography and ECG
C. Chest X-ray
D. CT Angiography
💬 Explanation: Echocardiography and ECG are standard tools to monitor phenotypic expression in mutation carriers.
17. Genetic counseling should be offered:
A. Only after genetic testing
B. Only to affected individuals
C. Both before and after genetic testing
D. Only when the test is positive
💬 Explanation: Genetic counseling is essential before and after testing to understand implications, risks, and inheritance patterns.
18. Which gene encodes cardiac troponin I?
A. TNNI3
B. TNNI3
C. TNNT2
D. MYH6
💬 Explanation: TNNI3 encodes cardiac troponin I, and mutations in this gene can be associated with HCM.
19. Which of the following best explains the concept of haploinsufficiency?
A. One functional copy of a gene is insufficient for normal function
B. Both alleles must be mutated for disease to occur
C. Mutation affects only females
D. Gene duplication enhances function
💬 Explanation: Haploinsufficiency refers to the situation where a single functional copy of a gene does not produce enough protein, leading to disease.
20. Which one of the following statements is true regarding HCM genetics?
A. All mutation carriers develop symptoms
B. Expression varies due to incomplete penetrance
C. Only men are affected
D. It follows mitochondrial inheritance
💬 Explanation: Due to incomplete penetrance and variable expressivity, not all individuals with a mutation will express the HCM phenotype.
11. Which of the following best describes the function of sarcomeric proteins mutated in HCM?
A. Ion channel regulation
B. Hormonal signaling
C. Force generation and contraction
D. Lipid metabolism
💬 Explanation: The mutated proteins in HCM are sarcomeric proteins responsible for force generation during myocardial contraction.
12. A variant of uncertain significance (VUS) in HCM genetic testing means:
A. No mutation is present
B. Diagnosis is confirmed
C. The clinical significance of the mutation is not yet known
D. The variant is benign
💬 Explanation: A VUS is a gene variant whose effect on disease risk is not clearly established and may need reclassification as evidence evolves.
13. What is the utility of cascade screening in families with known HCM mutations?
A. Confirms the diagnosis in the proband
B. Identifies other at-risk family members
C. Rules out other cardiomyopathies
D. Guides pharmacotherapy
💬 Explanation: Cascade genetic screening helps identify asymptomatic relatives carrying the familial mutation for surveillance and early intervention.
14. Which mutation is typically associated with late-onset hypertrophic cardiomyopathy?
A. MYBPC3
B. MYH7
C. TNNT2
D. TNNI3
💬 Explanation: MYBPC3 mutations are often associated with milder forms and later onset of hypertrophic cardiomyopathy.
15. Which of the following genes is NOT typically involved in hypertrophic cardiomyopathy?
A. MYH7
B. MYBPC3
C. CFTR
D. TNNT2
💬 Explanation: CFTR is not related to HCM; it is associated with cystic fibrosis. HCM involves sarcomeric protein genes.
16. Which clinical tool is commonly used to monitor mutation carriers in HCM?
A. Holter monitor only
B. Echocardiography and ECG
C. Chest X-ray
D. CT Angiography
💬 Explanation: Echocardiography and ECG are standard tools to monitor phenotypic expression in mutation carriers.
17. Genetic counseling should be offered:
A. Only after genetic testing
B. Only to affected individuals
C. Both before and after genetic testing
D. Only when the test is positive
💬 Explanation: Genetic counseling is essential before and after testing to understand implications, risks, and inheritance patterns.
18. Which gene encodes cardiac troponin I?
A. TNNI3
B. TNNI3
C. TNNT2
D. MYH6
💬 Explanation: TNNI3 encodes cardiac troponin I, and mutations in this gene can be associated with HCM.
19. Which of the following best explains the concept of haploinsufficiency?
A. One functional copy of a gene is insufficient for normal function
B. Both alleles must be mutated for disease to occur
C. Mutation affects only females
D. Gene duplication enhances function
💬 Explanation: Haploinsufficiency refers to the situation where a single functional copy of a gene does not produce enough protein, leading to disease.
20. Which one of the following statements is true regarding HCM genetics?
A. All mutation carriers develop symptoms
B. Expression varies due to incomplete penetrance
C. Only men are affected
D. It follows mitochondrial inheritance
💬 Explanation: Due to incomplete penetrance and variable expressivity, not all individuals with a mutation will express the HCM phenotype.
21. Which inheritance pattern is most commonly associated with HCM?
A. Autosomal dominant
B. Autosomal recessive
C. X-linked dominant
D. Mitochondrial
💬 Explanation: HCM is usually inherited in an autosomal dominant fashion with variable expressivity.
22. A pathogenic MYH7 mutation is most likely to affect:
A. Actin polymerization
B. β-myosin heavy chain
C. Tropomyosin alignment
D. Desmosomal proteins
💬 Explanation: MYH7 encodes the β-myosin heavy chain, a major sarcomeric protein frequently mutated in HCM.
23. Which gene mutation is most commonly found in patients with HCM?
A. MYH6
B. MYBPC3
C. TNNI3
D. ACTC1
💬 Explanation: MYBPC3 mutations account for a significant proportion of genetically confirmed HCM cases.
24. Penetrance in HCM refers to:
A. Proportion of mutation carriers expressing the disease
B. Degree of disease severity
C. Inheritance mode
D. Treatment response
💬 Explanation: Penetrance describes how many people with a mutation actually show symptoms of the disease.
25. Which of these is a characteristic of compound heterozygosity in HCM?
A. Only one gene is mutated
B. Different mutations on two alleles of the same gene
C. Multiple copies of the same mutation
D. Mutation affects mitochondrial genome
💬 Explanation: Compound heterozygosity involves having two different pathogenic variants at the same gene locus.
26. Which modality is first-line in family members of HCM patients?
A. Stress MRI
B. Genetic sequencing only
C. ECG and Echocardiography
D. Biopsy
💬 Explanation: ECG and echocardiogram are the initial modalities for screening relatives.
27. Modifier genes in HCM can influence:
A. Only inheritance
B. Disease expression and severity
C. ECG interpretation
D. Myocardial infarction risk
💬 Explanation: Modifier genes may affect phenotype variability among individuals with the same mutation.
28. A de novo mutation in HCM means:
A. Inherited from both parents
B. New mutation not inherited
C. Mitochondrial origin
D. Recessive trait
💬 Explanation: De novo mutations arise spontaneously and are not inherited from either parent.
29. Which concept explains why two individuals with the same mutation show different symptoms?
A. Genetic drift
B. Codominance
C. Variable expressivity
D. Incomplete dominance
💬 Explanation: Variable expressivity refers to different phenotypes seen in individuals with the same genotype.
30. Which of the following is an advantage of pre-symptomatic testing in HCM?
A. Early surveillance and risk stratification
B. Eliminates need for family screening
C. Confirms death risk
D. Prevents gene expression
💬 Explanation: Pre-symptomatic testing helps with early detection, lifestyle planning, and intervention.
31. What is the most common inheritance pattern of hypertrophic cardiomyopathy?
A. Autosomal dominant
B. Autosomal recessive
C. X-linked recessive
D. Mitochondrial
💬 Explanation: HCM is most commonly inherited in an autosomal dominant pattern with variable penetrance.
32. Which gene is most frequently mutated in patients with HCM?
A. TNNT2
B. ACTC1
C. MYBPC3
D. TPM1
💬 Explanation: MYBPC3 mutations account for a significant proportion of genetically confirmed HCM cases.
33. Which concept refers to the same mutation leading to different clinical outcomes in different individuals?
A. Genetic drift
B. Variable expressivity
C. Penetrance
D. Genetic anticipation
💬 Explanation: Variable expressivity refers to the range of symptoms expressed by individuals with the same mutation.
34. Which of these would be least likely to contribute to genetic heterogeneity in HCM?
A. Different mutations in the same gene
B. Mutations in different sarcomeric genes
C. Environmental modulation
D. Stable single-gene mutation
💬 Explanation: Stable mutations without variation are less likely to contribute to heterogeneity in phenotypes.
35. Which of the following is true about de novo mutations in HCM?
A. They can occur in individuals without family history
B. They always result in severe disease
C. They are not detectable on genetic testing
D. They occur only in females
💬 Explanation: De novo mutations arise spontaneously and may explain HCM in patients without a family history.
36. Incomplete penetrance in HCM means:
A. Everyone with the mutation shows symptoms
B. Not all mutation carriers develop the disease
C. The mutation affects only males
D. The disease always appears in childhood
💬 Explanation: Incomplete penetrance means that some individuals with a mutation may not express the HCM phenotype.
37. Genetic testing in HCM is especially useful for:
A. Confirming all cardiomyopathy diagnoses
B. Deciding on ablation therapy
C. Risk stratification and family screening
D. Determining exercise tolerance
💬 Explanation: Genetic testing is vital in HCM for identifying at-risk relatives and assessing disease expression and risk.
38. Which of the following best defines compound heterozygosity?
A. Two identical mutations in one gene
B. Two different mutations in the same gene
C. A mutation in mitochondrial and nuclear DNA
D. Mutation passed from non-biological parent
💬 Explanation: Compound heterozygosity means two different mutations in the same gene on each allele.
39. The term “phenocopy” in HCM refers to:
A. A non-genetic condition mimicking HCM
B. A genetic duplicate of the mutation
C. Monozygotic twin with mutation
D. Copy of genetic mutation on autosome
💬 Explanation: Phenocopies are conditions like athlete’s heart or Fabry disease that mimic HCM but have different etiologies.
40. What is the role of next-generation sequencing (NGS) in HCM?
A. Visualizing cardiac structures
B. Simultaneous analysis of multiple genes
C. Diagnosing arrhythmias
D. Directing surgical procedures
💬 Explanation: NGS allows rapid and cost-effective analysis of several genes linked to HCM, improving diagnosis and screening.
Genetics in Hypertrophic Cardiomyopathy (HCM)
🧾 20-Point Summary of Genetics in Hypertrophic Cardiomyopathy
- HCM is primarily autosomal dominant with variable penetrance.
- MYBPC3 and MYH7 are the most frequently mutated genes.
- Mutations affect sarcomeric proteins critical for myocardial contraction.
- Variable expressivity explains different clinical severity in mutation carriers.
- Penetrance increases with age in many cases.
- De novo mutations can cause HCM without family history.
- Genetic heterogeneity leads to diverse clinical presentations.
- Compound heterozygosity may worsen phenotype severity.
- Phenocopies mimic HCM but have different etiologies (e.g., Fabry).
- Next-generation sequencing (NGS) enables multigene analysis.
- Genetic counseling is essential before and after testing.
- Genetic findings aid risk stratification and cascade testing.
- Modifier genes and environment may influence outcomes.
- Mutations in TNNT2, TNNI3, ACTC1 are less common but relevant.
- Mitochondrial mutations are rare in classic HCM.
- Screening of first-degree relatives is critical.
- Some mutations associate with earlier onset and severe outcomes.
- Not all genotype-positive individuals are phenotype-positive.
- Interpretation of variants of uncertain significance (VUS) requires caution.
- Longitudinal follow-up of gene-positive, phenotype-negative individuals is important.
Genetics in Hypertrophic Cardiomyopathy (HCM)
🧠 Short-Answer Questions
- Describe the typical inheritance pattern seen in hypertrophic cardiomyopathy.
➡️ Answer: HCM typically follows an autosomal dominant inheritance pattern with variable penetrance. - Name two commonly mutated genes associated with HCM and their role in the myocardium.
➡️ Answer: MYBPC3 and MYH7 are commonly mutated; they encode sarcomeric proteins critical for myocardial contraction. - What is meant by “variable expressivity” in genetic diseases like HCM?
➡️ Answer: Variable expressivity means the same mutation can cause different clinical features or severities among individuals. - Explain the concept of incomplete penetrance in the context of HCM.
➡️ Answer: Incomplete penetrance means not all individuals with a pathogenic mutation will exhibit clinical signs of HCM. - How does genetic heterogeneity affect the clinical presentation of HCM?
➡️ Answer: Genetic heterogeneity leads to a wide variety of clinical outcomes due to mutations in different genes or variants. - What is a de novo mutation, and how can it lead to HCM in patients without a family history?
➡️ Answer: A de novo mutation is a new genetic change not inherited from parents, explaining HCM in sporadic cases. - What is the clinical significance of identifying compound heterozygosity in HCM?
➡️ Answer: Compound heterozygosity can lead to a more severe HCM phenotype due to two different mutations in the same gene. - Define a phenocopy and provide an example that could mimic HCM.
➡️ Answer: A phenocopy is a non-genetic condition mimicking HCM, such as Fabry disease or athlete’s heart. - What is the role of next-generation sequencing (NGS) in evaluating HCM?
➡️ Answer: NGS allows simultaneous analysis of multiple genes associated with HCM, improving diagnostic accuracy. - Why is genetic testing important for first-degree relatives of HCM patients?
➡️ Answer: It helps identify at-risk individuals, guides surveillance, and enables early detection or intervention.
Genetics in Hypertrophic Cardiomyopathy (HCM)
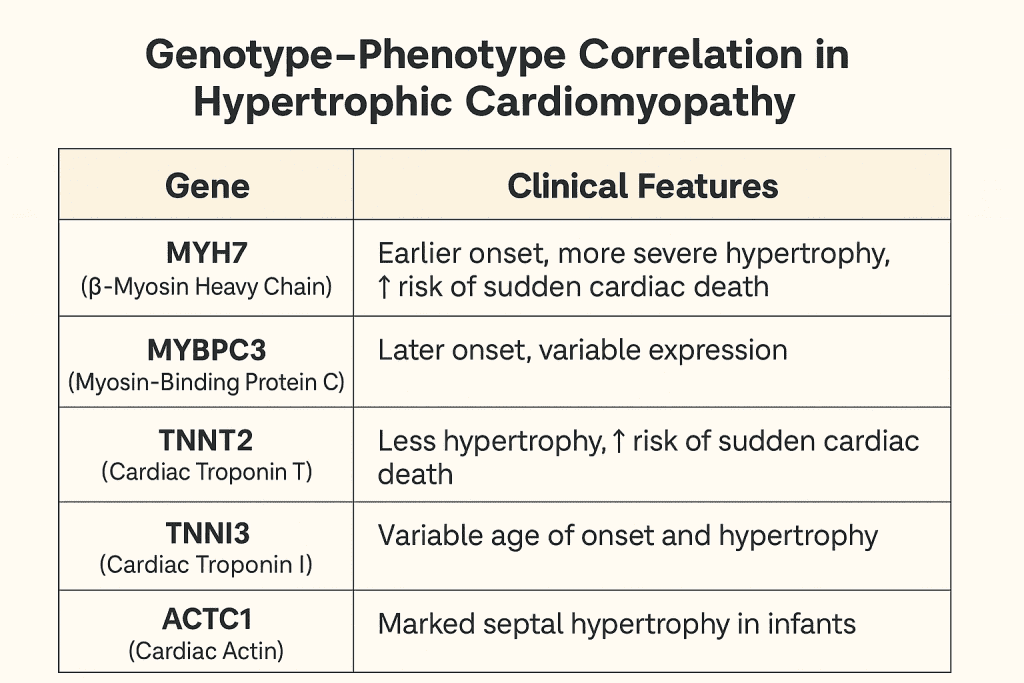
🧬 Genotype–Phenotype Correlation in Hypertrophic Cardiomyopathy
| Gene | Encoded Protein | Prevalence | Phenotype/Clinical Features |
|---|---|---|---|
| MYH7 | β-Myosin Heavy Chain | ~30–40% | Early onset, severe hypertrophy, increased risk of SCD |
| MYBPC3 | Myosin Binding Protein C | ~40–50% | Late-onset, variable penetrance, milder course |
| TNNT2 | Troponin T | ~5% | Minimal hypertrophy, high SCD risk |
| TNNI3 | Troponin I | ~3–5% | Mild hypertrophy, arrhythmias |
| ACTC1 | Cardiac Actin | Rare | Associated with apical HCM |
| TPM1 | α-Tropomyosin | Rare | Variable hypertrophy, conduction disease |
Note: SCD = Sudden Cardiac Death